Hip Implant Lawyers
Hundreds of thousands of patients have received hip replacements. The manufacturers of these devices have a duty to ensure that their products are safe and perform as advertised, and must warn doctors and patients of any safety risks they may pose. Those that don’t should not be surprised when they end up in court defending themselves against claims from patients who have been injured by their products.
According to the Centers for Disease Control (CDC), the number of hip replacements for patients aged 45 and over more than doubled between 2000 to 2010 from 138,700 to 310,800. The American Academy of Orthopaedic Surgeons (AAOS) reported that there were 370,770 total hip replacements in 2014.
Many thousands of people, unfortunately, have suffered from bad outcomes due to defective devices used in these surgeries. Consumernotice.org reported that between 2008 – 2017, there were 103,104 complaints to the FDA of hip replacements injuries. Several metal-on-metal hip implant devices have been recalled. As a result, according to Drugwatch, since July 2020 approximately 29,000 lawsuits have been filed against hip implant manufacturers by people suffering from serious complications from their hip replacement surgeries. These companies have paid more than $7 billion in settlements and verdicts to settle the cases against them. Recent jury verdicts have totaled over $1.7 billion. As of July 2019, more than 14,000 hip implant cases against multiple companies are still making their way through state and federal courts.
If you or a loved one underwent a hip replacement surgery and subsequently suffered pain and injuries related to the device, or have been told you need a revision surgery, you may be entitled to compensation. If so, Gerling Lawyers will be your zealous and powerful advocates who will cut through the red tape and maximize the compensation you deserve so that you can get your life back on track. Call the hip implant lawyers at Gerling Law today for a free, no obligation consultation.
What is Hip Replacement Surgery?
Arthritis in the hip is usually successfully treated with pain relievers, injections, and physical therapy, and lifestyle changes in its early stages. But, eventually, the damage worsens and the hip joint degenerates, causing these treatments to stop working and the pain may intensify for some people. For those patients who continue to suffer from chronic hip pain and who can no longer go about their daily activities, hip replacement surgery is usually recommended.
This procedure involves surgery whereby the hip is replaced with a synthetic one made of plastic, ceramic, and metal materials. During the surgery, the surgeon removes the damaged femoral head, which is the round part at the top of the thigh bone that fits inside the hip socket. A new, synthetic femoral “stem” is driven into the top of the thigh bone. A metal or ceramic “ball” or “femoral head” is placed on top of this femoral stem, replacing the round part of the bone that was removed. Next, the damaged cartilage is removed from the hip socket and replaced with a metal socket, called the “acetabulum.” The last step is the placement of a plastic, ceramic, or metal “spacer” between the new ball and socket to create a smooth gliding movement. This is done by using different components made by various manufacturers.
Hip resurfacing surgery is a similar procedure, but varies in that it involves leaving the femoral head in place, but trimming and capping it with a smooth covering so that it fits well inside the new hip socket.
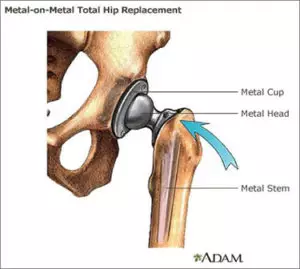
What are Metal-on-Metal Hip Implants?
Metal-on-Metal total hip replacement systems consist of a metal ball (femoral head), a metal femoral stem in the thigh bone, and a metal cup in the hip bone (acetabular component). The ball attaches to the taper of the stem.
Most large lawsuits against hip replacement manufacturers involve metal-on-metal designs. They were supposed to be more durable, but lawsuits claim the devices shed microscopic amounts of chromium, cobalt or other metals into the body.
Unlike hip replacement devices that place ceramic or plastic components next to the metal ones, MoM implants place metal components next to metal components, which over time, can result in fretting and corrosion. That corrosion can also cause metal ions to seep into the bloodstream, which can create a condition called “metallosis,” or metal poisoning, by releasing metal ions into the bloodstream. Metallosis also destroys bone, muscle and other tissue. In addition to creating immediate health problems for the patient, the condition can weaken an implant and cause it to fail. As the components move and slide against each other, they can shed tiny metal fragments into the surrounding tissue of the joint, causing severe adverse events like the following:
- Pseudotumors (pockets of fluid)
- Inflammation and swelling
- Tissue and bone death
- Metal poisoning
- Pain and difficulty walking or getting up from a seated position
- Weakening of the metal components
- Increased risk of hip loosening or dislocation
- Early implant failure requiring revision surgery
- Earlier than normal failure of the hip replacement
- Clicking, popping or grinding in the area of the hip implant
- Difficulty and pain when walking, standing or carrying weighted objects
- Leg length discrepancy
- Blood clots in the legs that can break free and travel to the lungs, heart, or in rare cases, the brain
- Infections at the incision site and deep tissue near the new hip
- Fracturing of hip bone
- Bone loss caused by device loosening (osteolysis)
- Dislocation or loosening of the implant in the hip joint
- Malalignment and loosening of the implant components
- Infection
- Severe pain
- Swelling of hip, thigh or groin area
- Blood toxicity from high levels of chromium and cobalt metal debris
- Metallosis (corrosion and fretting, metal flakes released into the bloodstream causing metal toxicity, pseudotumors, rash, necrosis or cardiac complications)
- Revision surgery required due to severe pain and local reaction (considerably longer recovery time than the initial surgery)
Physicians advise people who have undergone hip replacements and have, or think they may have, a MoM implant to contact their orthopedic surgeon, even if the joint appears to be functioning well, to rule out metallosis. Any of the above symptoms or related conditions can result in revision surgery to remove a hip device.
Ongoing Concerns with Metal-on-Metal Hip Implants
There has been controversy surrounding whether the benefit of metal hip implants outweighs the potential risks. In June 2012, the FDA held a two-day advisory panel meeting to discuss the thousands of adverse event reports received by the agency regarding MoM devices, results from medical studies and how to monitor the more than half-million U.S. patients with metal hip replacements. As part of its investigation, the FDA had asked manufacturers like Johnson & Johnson, Zimmer Holdings Inc. and Biomet Inc. to conduct long-term, follow-up studies of more than 100 metal-on-metal hips on the U.S. market.
The FDA determined in February 2016 that these devices should remain categorized as Class III (higher risk) devices requiring that premarket approval application (PMA) was needed to continue marketing them. To date, no MoM devices are marketed, but some patients who had hip replacement prior to May 18, 2016 may have received one.
In December 2017, the FDA issued a Safety Communication alerting the public about risks with MoM hip implants. The agency warned that some patients with these devices had developed an allergic reaction and experienced other medical problems such as:
- Neurological changes including sensory changes (auditory or visual impairments, nerve tingling, numbness, and damage)
- General hypersensitivity reaction (skin rashes)
- Tinnitus (ringing in the ears)
- Hearing and visual impairments
- Psychological status change (including depression or cognitive impairment)
- Kidney problems
- Thyroid problems (including neck discomfort, fatigue, weight gain, or feeling cold)
- Cardiovascular problems, including cardiomyopathy (disease of
the heart muscle)
Patients with MoM hip implants who experience hip/groin pain, noise, difficulty walking or a worsening of previous symptoms should see their orthopedic surgeon for further evaluation. Patients who experience new symptoms or medical conditions in their body other than at the hip should report them to their primary physician and remind them at that time that they also have a metal-on-metal hip implant.
Rushing Products To Market
A problem at the heart of the issue with MoM implants was a common one among certain manufacturers and medical devices — the lack of clinical studies confirming their safety before the FDA approval. This was due to the 510(k) Pre-market Notification fast-track bypass of the usual approval process if the manufacturer could show that the device was “substantially equivalent” to one already cleared by the FDA, allowing it to rush its product to the market. Several hip implant manufacturers did just that and we discovered years later that their products were, in fact, harmful and defective. But, until then, they had reaped millions in profits to the detriment of innocent patients. To recover for the damages they have received through no fault of their own, thousands of patients have filed hip implant replacement lawsuits against these manufacturers, in some cases creating large consolidated multidistrict litigations (MDLs) where one judge oversees the pre-trial proceedings. Some of these litigations have already resulted in large settlements for patients.
Types of hip replacement systems that have been identified with increased risks of complications, or that have been recalled because of higher-than-normal revision rates, are listed below:
- Zimmer: Durom Cup hip (recalled in 2008);
- Johnson & Johnson/DePuy: ASR Total Hip Replacement and ASR Resurfacing System hip (recalled on August 24, 2010);
- Stryker: Rejuvenate and ABG II Stems (recalled on July 4, 2012);
- Biomet: M2a and 38 Diameter hips,
- Wright: (a) Conserve, (b) Dynasty, (c) Lineage and (d) Profemur (femur fracture) hips, and
- Smith & Nephew.
Hip Replacement Manufacturer Lawsuits in 2022
As a result of the heavy scrutiny these hip implant manufacturers have been under over the past several years regarding the dangers of their MoM hip devices, many lawsuits have been filed against them which have been settled in recent years to resolve the claims of severe complications caused by the devices.
Zimmer Holdings
Makers of the Durom Cup hip replacement system, Zimmer Holdings, the country’s largest artificial joint component manufacturer, was one of the first hip replacement companies, along with DePuy, to face the consequences of designing a defective product. Zimmer’s Durom Acetabular Component, more commonly referred to as the Durom Cup, arrived on the market in 2006. Not long thereafter on July 24, 2008, they announced that they were recalling it because of numerous reports of loosening, later requiring revision surgery. They created a new surgical training program, then returned the product to the market with updated instructions. Zimmer later paid millions of dollars in compensation for injuries. As of July 2019, there were 51 lawsuits still pending in the Durom Cup MDL. More recently, Zimmer has faced lawsuits over different versions of its M/L Taper Hip Prosthesis implanted with Zimmer’s Versys Femoral Head. The federal panel combined those lawsuits in an MDL in October 2018. As of July 2019, there were 113 lawsuits pending in the mass litigation.
DePuy Orthopaedics – A Johnson & Johnson Company
Makers of the Pinnacle and the ASR XL Acetabular and Resurfacing MoM Systems, DePuy is a subsidiary of Johnson & Johnson (J&J), and has had to defend thousands of lawsuits concerning these two hip replacement devices. The ASR devices were recalled in 2010. Data shows that approximately 13% of patients who received a DePuy ASR hip implant had to undergo a second hip surgery to remove the defective implant. Between 2013 and 2015, Johnson & Johnson settled litigation of 9,800 lawsuits involving its ASR MoM hip device for $4.2 billion, but the MDL remains active in the Northern District of Ohio. The Pinnacle devices were discontinued in 2013 after being found to discharge metal particles into a patient’s bloodstream. After having hundreds of millions in verdicts rendered against it, as of July 2019, there were still 9,893 lawsuits pending against DePuy in the Northern District of Texas.
Wright Medical
Makers of the Conserve and Profemur hip replacement systems, Wright’s products have also been linked to serious complications like instability, metal toxicity, pain and immobility, and premature failure. Wright had the same issues with them as the other device manufacturers had. While the Conserve was a MoM design with all the usual problems, the Profemur was linked to higher-than-expected rates of revision surgery because the femoral neck was vulnerable to degradation, fractures, and breaks. In 2012, lawsuits over Wright Medical’s Conserve implants were combined into an MDL. Wright Medical agreed to two settlements totaling $330 million to resolve hundreds of lawsuits over its hip-replacement implants. After the final settlement, a federal court closed the multidistrict litigation against Wright in June 2018. The company no longer makes hip implants.
Wright hips named in lawsuits:
- Conserve Total Hip Implant System
- Conserve Total A-Class Advanced Metal Hip Implant System
- Conserve Resurfacing System
- Profemur System
- Dynasty hip implants
- Lineage hip implants
Biomet
This manufacturer initially advertised their M2a Magnum MoM hip implant as ideal for younger individuals because it was supposedly more durable. By the time it had been on the market for several years, however, hundreds of patients had filed reports of problems with the device. It was never recalled, but Biomet has had to defend numerous lawsuits concerning the device. Lawsuits over Biomet’s M2a Magnum implants were combined into an MDL in 2012.
Stryker Orthopedics
For many years, Stryker Orthopedics has been defending hundreds of lawsuits targeting their hip replacement systems. At least six models have been named in lawsuits. Three brands — Rejuvenate, ABG II, and LFIT Anatomic CoCr V40 femoral heads — have been included in federal mass litigations. Stryker began marketing its Rejuvenate Modular Primary Hip System in June 2008. Four years later, July 6, 2012, Stryker announced a voluntary recall of both the Rejuvenate and ABG II Modular Hip Systems because of complications associated with the modular-neck stems. Fretting and corrosion which develops at the modular neck interface lead to significant amounts of dangerous cobalt and chromium metal ions being released into surrounding tissues and the bloodstream. These metal ions in the body lead to serious medical problems such as tissue necrosis and pseudotumors and forced some individuals to have to undergo another hip replacement surgery.
Numerous lawsuits allege that Stryker was negligent in failing to warn the public about the high rate of failure. They also accuse the company of making fraudulent claims, stating that the titanium and cobalt chrome neck and stem in the Rejuvenate and ABG II systems were resistant to fretting and corroding which has been proven not to be true. In 2013, the judicial panel combined the lawsuits over Stryker’s Rejuvenate and ABG II into an MDL.
In November 2014, Stryker Corporation and its subsidiary, Howmedica Osteonics Corporation, reached a $1 billion deal to settle thousands of New Jersey and Minnesota claims involving these two systems. As of April 2019, there were 1,216 lawsuits pending in the District of Minnesota with the MDL remaining active.
In 2016, their LFIT Anatomic CoCr V40TM femoral heads received higher than normal complaints of taper lock failure—weakening and corrosion around the junction between the femoral stem and the femoral ball. Stryker released an urgent medical device notification recalling certain sizes of this component. Hundreds of people filed lawsuits over the company’s LFIT V40 components after Stryker recalled 42,519 units, citing “higher than expected” complication rates due partly to its failure to lock into the hip socket properly. The issue caused severe complications including excessive metal debris wear from the cobalt-chromium head that entered the bloodstream, resulting in metallosis. Other issues included spontaneous dissociation, where the femoral head disconnected from the hip due to metal corrosion, along with the need for complicated revision surgery to replace the device.
In 2014, Stryker had agreed to pay $1.43 billion to settle thousands of cases. It extended that initial settlement to more patients in 2016, raising the total settlement amount to around $2 billion. The average amount paid for each problematic hip implant was $600,000. Under the settlement, the deadline to file a lawsuit over those hip replacements was March 2017. Stryker expected all settlement payments to be delivered by the end of 2017.
In 2017, the MDL panel combined the first federal lawsuits over Stryker LFIT V40 femoral heads into an MDL in Massachusetts federal court. New Jersey followed suit a month later, combining state lawsuits over the device into an MCL in state court. Stryker agreed to a confidential settlement of lawsuits over its LFIT V40 Femoral Head on November 2, 2018. As of April 2019, there were still 662 pending in the District of Massachusetts in the settlement phase. In all, as of August 2019, there were nearly 2,000 Stryker hip replacement lawsuits in state and federal courts.
Currently, attorneys are investigating and have begun accepting cases involving complications related to Stryker’s Tritanium Acetabular Shells. At this point, it is unknown how many lawsuits there will be because the litigation is in its early phases with the location yet to be determined.
Smith & Nephew
Makers of the R3 Acetabular hip replacement system, Smith & Nephew also received reports of higher-than-expected rates of revision surgery associated with their device. In this case, it was the metal lining at the top of the femoral head that was rubbing against the ball component and creating corrosion. The company recalled this system in 2012.
The BHR System was recalled in June 2015 due to studies showing the device was causing a high-rate of complications which required revision surgery in patients.
The Birmingham™ Hip Resurfacing (BHR) System is a metal on metal hip implant that was used in patients who needed partial hip resurfacing. The BHR implant consisted of a spherical metal cap on the femur and metal cup installed within the hip socket. The Birmingham™ Hip Resurfacing (BHR) System was implanted in patients from 2006 to 2015. Reported adverse effects include:
- Metallosis
- Pseudotumors
- Revision surgery
- Dislocation
- Fractures
- Necrosis
- Chronic pain
- Inflammation
Later, in 2016, they set out an urgent field safety notice also recalling their Modular SMF and Modular REDAPT Revision femoral hip systems because of higher than anticipated complaints. The U.S. Judicial Panel on Multidistrict Litigation grouped the first Smith & Nephew lawsuits into a single litigation in 2017. The cases are still in the early stages. As of July 2019, 587 were still pending in the District of Maryland in the pre-trial stages.
Over $500 Million in Recoveries
I called my insurance company to start my claim – then I immediately called Gerling. They know the laws; they know the ins and outs. If you want something done – and done right – call Gerling. They know what they are doing.
Gerling Law Works on a Contingency Basis
Gerling’s experienced hip replacement lawyers work on a contingency basis, which means there are no upfront costs. We only get paid if you receive money.
We Handle Mass Torts All Over The Country
We consider ourselves a national law firm that represents plaintiffs in mass tort cases in federal and state courts across the country. We have attorneys admitted to the full time practice of law in Indiana, Illinois and Kentucky, and we can be admitted to practice on individual matters in other courts and, if necessary, we can engage local counsel at our own expense to comply with state ethical rules. In short, our mass tort clients live all over the country. No matter where you reside, we can help you with your case.
We Do The Work For You
We minimize paperwork, stress, and wait time as much as possible.
Over 50 Years Experience
Our lawyers have helped thousands of injured victims and their loved ones.
What’s The First Step?
If you or a loved one has been injured by a metal-on-metal hip replacement, you may have a claim against the manufacturer. If you received a metal hip implant and have experienced any of the symptoms listed above, contact your physician immediately. Also, do not release any medical information to the hip replacement manufacturer representatives or sign a release before speaking to a lawyer. If you are told that you need hip revision surgery, make sure that you communicate clearly to the surgeon that any and all removed component parts are to be preserved and consult with your counsel to ensure that they are saved properly.
The first step in the legal process is to contact a hip implant lawyer for a free consultation. Gerling Law’s staff of attorneys, paralegals, and case support personnel are skilled at providing the extraordinary personal attention that your lawsuit deserves. Your lawyer will review your work history, medical history and other facts pertinent to your case and advise you on your eligibility to participate in a hip implant lawsuit. Contact Gerling Law today for a free, no-obligation lawsuit consultation.