Bard IVC Filter Lawyers
Am I Eligible To Participate In An IVC Filter Lawsuit?
If you or a loved one has suffered complications after receiving an inferior vena cava (IVC) filter, you may be entitled to compensation from a lawsuit filed against the manufacturer. The Food and Drug Administration (FDA) received 921 reports of adverse events related to these filters between 2005 and 2010. There is a serious risk of these blood clot filters breaking apart and perforating patients’ veins, or migrating to the heart, lungs, and other major organs, complications that can put patients at risk for serious injury or death. Thousands of lawsuits have been filed seeking to hold the manufacturers of these devices accountable for the injuries caused by their disregard for the health and safety of patients. These device manufacturers have a duty to ensure that their products are safe and perform as advertised, and must warn doctors and patients of any safety risks they may pose. Those that don’t should not be surprised when they find themselves in court defending claims by patients who have been injured by their products. If you or someone you love had an IVC filter implanted and later suffered a major complication related to the device, you may be entitled to compensation. If so, let the IVC filter lawsuit attorneys at Gerling Law cut through the red tape and maximize the compensation you deserve so that you can get your life back on track. Call the IVC filter lawyers at Gerling Law today for a free, no-obligation case evaluation.
What Is An IVC Filter?
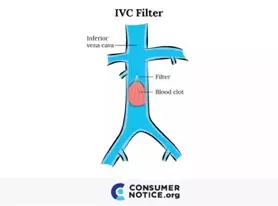
An IVC filter, or a blood clot filter, is a small cage-like device used for the prevention of pulmonary embolism by trapping blood clots as they travel through the inferior vena cava (the major blood vessel returning blood from the lower half of the body to the heart) toward the heart and lungs. Invented in the mid-1960s, the first surgical placement of an IVC filter took place in 1967. Since then, the IVC filter has evolved over the years. Multiple models have been created with different materials and shapes, using various insertion and retrieval methods. One of the most common IVC filters, the Gϋnther Tulip®, was first approved by the FDA in 2003. Since then other models have been approved and thousands of people have had IVC filters surgically implanted.
Why Do Doctors Use IVC Filters?
Doctors use IVC filters when patients cannot be treated with blood thinners. Usually, these filters are inserted into the inferior vena cava vein in people with deep vein thrombosis (DVT or risk of DVT). IVC filters come in many different shapes, but they all do basically the same thing: catch and break up blood clots as they travel through the bloodstream before they reach the lungs. A clot in the lungs can cause a pulmonary embolism, which could damage the lung and other organs. Some of these filters are designed to have the option to retrieve them at a later date. The option to retrieve models are designed to be relatively easy to remove when the patient is no longer at risk for future blood clots. Unfortunately, while permanent IVC filters with the option to retrieve can catch and break up blood clots, they can also cause severe complications.
What Are The Complications Of Permanent IVC Filters?
Before your IVC filter implant procedure, your doctor may have warned you about some potential risks, such as an inability to stop the blood clots. However, you may not have been alerted to the severe complications these filters can cause. Some of these result in serious health problems, including severe and chronic pain in the chest or heart, pulmonary embolism, respiratory problems, and death. Adverse side effects that have been linked to vena cava filter (IVC) devices include:
- Filter migration
- Filter fragmentation and embolization (movement of the entire filter or fracture fragments to the heart or lungs)
- Damage to organs
- Perforation of the IVC
- Filter fracture
- Filter failure
- Pulmonary embolism
- Tilting of the filter
- Irretrievable filters
- Difficulty removing device
- Cardiac tamponade (fluid build up)
- Deep vein thrombosis (DVT)
- Emergency removal of a device
- Hemorrhage
- Perforation of tissue, vessels, and organs
- Respiratory distress
- Severe pain
- Shortness of breath
- Death
FDA Warnings
Since the introduction of the IVC filter, the FDA has received thousands of adverse reports involving these devices. Specifically, it was reported that the IVC filters break off and cause blockages in the body. The FDA has issued several warnings about the potential side effects of IVC filters, the first in 2010, at which time the agency reported that it had received more than 900 reports of adverse events involving IVC filters since 2005, including 328 reports of device migration, 146 reports of filter component detachment, 70 reports of a perforated vein or organ, and 56 reports of filter fracture. The FDA first released a public safety communication in August of 2010, warning of possible adverse health events associated with long-term IVC filter use. It recommended that if a patient has a permanent IVC filter with the option to retrieve, the patient’s individual risk/benefit profile should be examined and the filter should be removed as soon as medically safe to do so. According to the FDA, the IVC filters are intended for short-term placement and have caused risks when left in long-term, including lower limb deep vein thrombosis (DVT), filter fracture, filter migration, filter embolization, and IVC perforation. A number of studies published indicated that IVC filter complications occurred more frequently than previously expected. In 2012, the American Journal of Medicine published an IVC filter study, indicating that the filters only benefit a small percentage of pulmonary embolism patients and exposed the rest to a potential risk of side effects. The following year, the FDA issued an updated warning following its earlier 2010 public safety communication about the medical devices. This time it narrowed its recommendation that doctors remove retrievable IVC filters as soon as possible after the risk of pulmonary embolism to a recommendation of removal 29 and 54 days after implementation. Unless there was a very good reason to keep the IVC filter in place, the FDA and Radiological Society of North America recommended the removal of retrievable IVC filters once the danger of a life-threatening clot is over. However, according to another study published in JAMA in 2013, only about 8.5% of retrievable IVC filters were removed successfully. Despite FDA warnings, researchers discovered only 58 out of 679 (less than 10%) of retrievable IVC filters inserted were actually removed. Other results discovered a majority of the filters remained in patients longer than medically necessary with the following results:
- 3 percent failed attempts at removing the filters
- 8 percent had venous thrombotic events
- Approximately 25 of those events were pulmonary embolisms
Cordis IVC Filter Recall
In 2013, the FDA announced a recall of all Cordis retrievable IVC filters manufactured citing an issue with the device’s labeling and implant instructions. Around 33,000 devices were recalled, all of which were distributed between May 6, 2010, and April 2, 2013. In 2014, the FDA released a second public safety communication, warning of further adverse health events associated with long-term IVC filter use, as well as problems with specific products. The safety communication explained that the FDA had concerns that retrievable IVC filters, purposed for temporary use, are not always removed within the appropriate time span. “For patients with retrievable filters, some complications may be avoided if the filter can be removed once the risk of pulmonary embolism has subsided.”
C.R. Bard Warning Letter
In 2015, C.R. Bard came under fire after an FDA investigation found that the manufacturer was producing a certain IVC filter device removal system without marketing clearance or approval. As a result, the FDA sent a warning letter to C.R. Bard notifying the company that they were in violation and subject to penalization. The warning letter accused the company of making and selling two IVC filter retrieval devices that had been neither approved nor cleared by the FDA:
- Recovery Cone Removal System, Model RC-15, and
- Recovery Cone Removal System, Model FBRC.
Perhaps more importantly, though, the FDA’s warning letter accused Bard of failing to properly report IVC device malfunctions that contributed to a death or serious injury in the MAUDE database. Each of these failures drastically understated the severity of the device malfunction in Bard’s favor, covering up the dangers of IVC filters. Despite these FDA warnings to the manufacturers about the problems with IVC filters, these devices continue to be implanted in thousands of patients every year. According to the results of a new study published in a 2019 American Journal of Cardiology report, despite clinical trial data showing IVC filters are ineffective for reducing mortality following deep vein thrombosis (DVT), the proportion of patients with DVT given an IVC filter continued to rise over an extended period of time.
Recalls for IVC filters
The FDA has never issued a mandatory recall of an IVC filter implant. IVC filter manufacturers have made several voluntary recalls of IVC filter models that have been especially dangerous. However, none of those voluntary recalls have gone far enough in removing defective and dangerous products from the market. The table below provides the IVC filter recall list, updated through May 2019:
Overuse and Improper Use By Doctors
A 2016 study published in the American College of Cardiology cited evidence that found that IVC filters were being over-used by doctors. The study concluded that doctors were using IVC filters for a variety of medical purposes, despite the lack of information regarding appropriate use and surveillance of the filters. The study also found retrievable rates to be lower than expected. As of 2020, it was estimated that 259,000 filters were employed and the market value of vena cava filters continues to increase despite FDA warnings. Today, the IVC filter market is worth $656 million and is growing rapidly.
Manufacturers of IVC Filters
IVC filter lawsuits claim that the devices were defective, making them more likely to fracture or perforate the inferior vena cava. The manufacturers who have been sued are:
- C.R. Bard
- Cook Medical
- Cordis
- Argon Medical
Additional manufacturers are listed below:
- Boston Scientific
- Braun
- ALN
- Rafael Medical
- Volcano Corporation
- Rex Medical
IVC Filter Lawsuits in 2022
IVC filters have been marketed as a means of protecting patients from dangerous blood clots, but what many IVC filter patients are discovering is that the devices can fracture, migrate and puncture the inferior vena cava, which has prompted the growing number of blood clot filter lawsuits against filter manufacturers.
Patients are claiming that the manufacturers of these filters have not addressed safety concerns but, instead, continue to charge forward with sales of these filters despite knowledge of their serious dangers and failures. Consequently, thousands of lawsuits have been filed and continue to move forward against IVC filter manufacturing companies.
The lawsuits currently pending against C.R. Bard, Cook Medical, and other IVC filter makers allege that the manufacturing companies knew, or should have known, about the problems with their medical devices, yet failed to properly warn healthcare providers and their patients about the risks of IVC filter placement. The plaintiffs in the IVC filter litigation seek to hold the device makers accountable for their adverse health events, which they allege are due to defects in the design of certain IVC filter devices, and juries have already awarded millions of dollars in damages to IVC filter recipients.
These pending lawsuits claim that the adverse health events associated with retrievable IVC filters are the result of the manufacturer’s negligence, defective design in the product, manufacturing defects, failure of the manufacturer to adequately warn consumers of risks, breach of an implied warranty, and misrepresentation of the product by manufacturers and their subsidiaries.
The Judicial Panel on Multidistrict Litigation combined the lawsuits against each company into multidistrict litigations (MDLs) in order to move the lawsuits through the legal process more efficiently. The lawsuits against Cook Medical were consolidated into an MDL in the U.S. District Court for the Southern District of Indiana (MDL No. 2570), the lawsuits against C.R. Bard were centralized in the U.S. District Court for the District of Arizona (MDL 2641), and the lawsuits against Cordis were consolidated in the Superior Court of California, County of Alameda in 2019.
Bellwether trials — representative test cases used to determine possible settlements — had begun in both MDLs as of July 2018. Neither Bard nor Cook Medical had offered a global settlement. The companies had agreed to a few individual settlements for undisclosed amounts.
As of November 2020, more than 9,400 lawsuits had been filed against two of the IVC filter makers. Cook Medical faced 7,401 lawsuits in an Indiana federal court. Bard faced another 2,083 in an Arizona federal court.
IVC filter attorneys believe hundreds of more people could file suits. Currently, no IVC filter class-action lawsuits have been filed in the United States. Law firms launched at least two class actions in Canada, both targeting Cook Medical’s IVC filters.
Filing an IVC Filter Lawsuit
There are some important steps you should take before beginning the IVC filter lawsuit process. First, you should try to mitigate your injuries and damages by seeking proper medical treatment, including getting second opinions, if desired, and following your doctor’s instructions. You should also familiarize yourself with the civil litigation process so you know what to expect of your IVC filter lawsuit. Next, you should retain your IVC filter lawyer to represent you. Third, working with your lawyer, you should learn about the lawsuit process to familiarize yourself with the important timelines and procedures of an IVC filter lawsuit. Each state has its own deadline, or statute of limitations, for allowing victims of defective medical devices to file these kinds of lawsuits. Lastly, together with your lawyer, you should begin to gather your evidence that you already have in your possession that supports your claim. This may include, but is not limited to, medical records, work records, your doctor’s expert testimony, your and your loved ones’ personal testimony, and bills associated with your injury.
How An Experienced IVC Filter Lawyer Can Help
Cases involving defective medical devices are complex and need in-depth expertise of the legal issues involved. An experienced, knowledgeable attorney knows how to thoroughly investigate the product’s safety and identify all of the potentially responsible parties, along with their insurance companies. Then the attorney will work with medical experts to build a compelling case to support and establish your right to receive maximum compensation for your injuries from the at-fault parties and their insurance companies. If you have suffered a disabling injury, you may require ongoing medical care and physical therapy. The future costs of your injury should be considered as well as the costs to date. We work with a life care planner to develop an estimate of your future medical expenses and ensure they are taken into account in any settlement. Unfortunately, in dangerous medical device cases, it is common for numerous people to have been harmed before the danger of the product comes to light. Evidence in a faulty medical device case may come from an FDA action, such as a recall, from independent research begun after problems are publicized, or from manufacturers’ documents about the device and its testing, which a court has ordered released. A dangerous medical device may eventually be the subject of multiple lawsuits backed by evidence of its harm to patients and the manufacturer’s liability. If numerous suits are filed, the courts may combine similar claims to make them easier to manage and process. There are multiple ways in which these types of lawsuits might be handled. The two most common include 1) class action litigation; and 2) multidistrict litigation (MDL). When cases that share common questions of fact, legal issues, and injuries are pending in different federal district courts, they may be transferred to any district for consolidated pretrial proceedings so that they can be handled more efficiently. The cases will still be tried separately. These transfers are made by the judicial panel on multidistrict litigation authorized by 28 U.S.C. 1407 and combined as multidistrict litigation (MDL) which consolidates similar cases, sometimes hundreds or thousands, throughout the country that involve the same product. Often, large medical device manufacturers who are facing multiple lawsuits will set aside millions or billions of dollars in anticipation of paying settlements if a deal can be worked out. A Gerling Law dangerous medical device lawyer who is familiar with these legal actions can help to explain how an injured individual’s case might proceed.
Over $500 Million in Recoveries
I want to thank my attorney, Jesse Poag, and paralegal, Christina Von Zirkelbach. They were awesome at answering any questions I had while assisting me in getting qualified for disability benefits. Thank you, Jesse and Christina- you’re awesome! – Susan G.
What’s the First Step?
If you or a loved one has been injured by an IVC filter, you may have a claim against the manufacturer. The first step in the legal process is speaking with an IVC filter lawsuit attorney. The Gerling Law team’s staff of attorneys, paralegals, and case support personnel are skilled at providing the extraordinary personal attention that your lawsuit deserves with over 50 years of experience helping clients get the justice they deserve. Your lawyer will review your work history, medical history and other facts pertinent to your case and advise you on your eligibility to participate in an IVC filter lawsuit.
Contact Gerling Law today to learn how we can help with a free, no-obligation consultation. There is no charge unless we help you recover money. The Gerling Law team will always work diligently and efficiently in handling all aspects of your claim so that you can focus only on restoring your health and getting your life back on track.